Periodycal #13: Flower pHun & cosmetic concerns
The chemistry behind the colours of your garden's blooms, and assessing cosmetic ingredient safety
Welcome to another edition of the Periodycal newsletter! I had gap between newsletters but back this week with some summer-themed chemistry as the warmer months approach in the northern hemisphere. There’s a look at the chemistry that colours summer blooms, as well as a reminder of the various natural indicators you can make from flowers at this time of year. On a different note, the latest edition of Periodic Graphics in C&EN looks at cosmetic chemical concerns. Finally, there are the usual round-ups of upcoming topical chemistry tie-ins and interesting chemistry news and features.
Summer flower chemistry
The fleeting blooming season of peonies is here, heralding the start of a few months of colourful flowers in our gardens. As well as peonies, coloured by anthocyanins including peonin, there are roses, which owe their hues to carotenoids and anthocyanins, and later in the summer dahlias, coloured by a combination of anthoycanins and other pigments. The latter graphic also explains why we don’t see blue varieties of these flowers – a quirky biochemistry omission that leaves them unable to make a certain pigment.
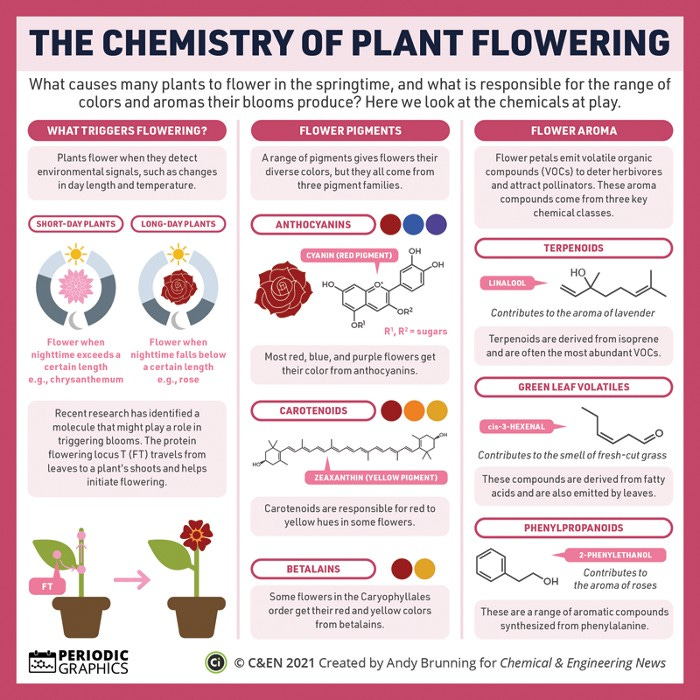
For more on the chemistry that kicks blooming off in the first place, this graphic in C&EN (below) provides a handy summary. And if it’s the smells that interest you more than the colours, this graphic picks some key aroma compounds from a selection of popular flowers.
‘Tis the season for garden indicators
Flowers aren’t only good for brightening your garden – they’re also a novel way to produce pH indicator solutions! You might remember that I’ve previously highlighted this project from Isobel Everest to catalogue the different coloured indicators that can be made from various garden plants. The project continues to grow, and you can join in using the #GardenIndicators tag on Twitter. Not sure how to get started? I wrote a brief blog on the basics as part of the day job here.
Cosmetic chemistry concerns
The latest edition of Periodic Graphics in C&EN takes a look at the topic of cosmetic ingredients. Compounds such as phthalates and parabens have often been subject to concerns around their use, but the issue of cosmetic ingredient safety is rarely a black and white one. This graphic summarises some of the evidence surrounding these components to give a balanced picture of how concerned we should be.
Upcoming chemistry tie-ins
Here’s a quick run-down of upcoming events or days and links to some relevant chemistry graphics from the archives:
1 June: National Nail Polish Day — The chemistry of nail polish
1 June: World Milk Day — Why is milk white? and How do plant milks compare to cow’s milk?
2 June: National Running Day — The materials science of athletics tracks
3 June: World Bicycle Day — The materials science of cycling
4 June: National Cheese Day — The science of making cheese
8 June: World Oceans Day — Ocean acidification
10 June: World Gin Day — The chemistry of gin (and tonic)
Chemistry news and features
Here’s the regular selection of chemistry news and features I’ve found interesting over the past couple of weeks:
Swapping out silicone in our skin and hair products — This article in C&EN ties in with this month’s edition of Periodic Graphics and looks at how silicones are being increasingly phased out of personal care products and the challenges of replacing them.
Potential antidote for deathcap mushrooms found — The deathcap mushroom is responsible for most deaths from mushroom poisoning worldwide. Now, researchers have identified a dye that appears to block its toxic effects.
Researchers use AI to discover new antibiotic — New families of antibiotics have been famously hard to come by over the past couple of decades. It’s fantastic news for the battle against antibiotic resistance if researchers can use AI to identify new potential antibiotics faster.
That’s all for this edition of the newsletter. Got ideas for future graphics or newsletters? Drop them in the comments below!
Thanks for reading,
Andy








Can we get an updated Plant Milks infographic for June with all figures normalized to kcal rather than ml? This would be a more relevant comparison considering food consumption recommendations are based on energy intake rather than volume of food.